
Looking for the right phase for your chromatographic application? Correct phase selection is a trial-and-error process, but fortunately for you, many of those trials (and errors!) happened long ago.
Selecting the right phase demands consideration of the application, column chemistry, separation mode and particle morphology.
In this post, we'll share a basic roadmap for chromatographers to follow in order to accelerate your efforts and (hopefully) reduce or eliminate tedious guesswork.
Step One: Choose the Particle Morphology
When it comes to packing materials, size, shape and structure matter.
- With silica, spherical particles yield better results than irregular particles — specifically: sharper peaks, higher efficiency, and increased resolution owing to their more uniform packing characteristics.
- Smaller particles yield more efficient and higher resolution separations, but as particle size decreases (for example from 10 to 7, 5 or 3µm diameters) there is an exponential increase in backpressure.
- Pore size selection should match the size of the analyte. Compounds are generally divided into one of two categories, those with a molecular weight below 5,000-10,000 Daltons (<5-10 kDa, or ‘low molecular weight' compounds), and those with a molecular weight greater than 5,000-10,000 Daltons (>5-10 kDa, or ‘high molecular weight' compounds). It is an important distinction, since the packing material pore size should match the analyte's in order to optimize interactions with the stationary phase: low molecular weight compounds are best suited to packing materials with small pore sizes, while larger molecules demand wider-pore packing materials. Here's a general guideline:
Analyte Type Ideal Pore Size in Angstroms (Å) Organic molecules 30 - 70 Polypeptides & most proteins 90 - 300 High molecular weight proteins 500 - 1,000+
Below are two charts that can help you narrow down the right pore diameter and particle size when searching for the most suitable sorbent, based on sample molecular weight and generated pressure:
Selecting Pore Diameter
| Sample Molecular Weight (MW in Dalton) | ||||
| ▼ | ▼ | ▼ | ||
| Organic Molecules | Peptides | Proteins (Polypeptides) Bio-polymers |
||
| ▼ | ▼ | ▼ | ||
| MW < 1,000 Dalton | 1,000 < MW < 10,000 Dalton | MW > 10,000 Dalton | ||
| ▼ | ▼ | ▼ | ||
| 30 Å, 40 Å (< 400 Dalton) 60 Å, 70 Å (400 - 1,000 Dalton) |
90 Å, 100 Å 120, 150, 300 Å |
500 Å, 800 Å, 1,000 Å | ||
Selecting Particle Size
| Generated Pressure (GP) or Selectivity (α) | ||||||
| ▼ | ▼ | ▼ | ▼ | |||
| GP ~950 psi (65 bar) α < 1.2 (very difficult separation) |
GP ~220 psi (15 bar) 1.2 < α < 1.5 (difficult separation) |
GP ~73 psi (5 bar) 1.5 < α < 2.0 (possible separation) |
GP ~29 psi (2 bar) α > 2.0 (easy separation) |
|||
| ▼ | ▼ | ▼ | ▼ | |||
| From 0 to 30 μm | From 15 to 40 μm | From 40 to 100 μm | From 40 to 1,000 μm | |||
Step Two: Choose the Separation Mode
You'll need to choose a separation mode based on the polarity and solubility of the analyte. Analytes which are soluble in water and polar solvents typically use reversed-phase, ion exchange or HILIC separation modes. For those analytes using non-polar solvents, normal or reversed-phase techniques are appropriate.
Here's a quick reference summary list:
- Normal Phase (NP): Normal Phase chromatography uses a polar stationary phase and a water-immiscible non-polar mobile phase. The polar functional groups of the analyte are retained via polar interactions with the polar groups of the stationary phase, and then eluted from the column according to increasing polarity. With silica gel stationary phases, these interactions typically occur on the silanols of the silica surface which are highly polar. Normal Phase is effective in the separation of analytes possessing low to intermediate polarity, though it is a less effective technique for water-soluble analytes.
- Reversed-Phase (RP): In Reversed-Phase chromatography, the opposite configuration of Normal Phase is used. With a non-polar stationary phase and a polar mobile phase, analytes with higher hydrophobicity are retained. As packing material hydrophobicity increases, the retention of non-polar analytes grows.
- Ion Exchange (IEX): Ion Exchange Chromatography relies on surface charge to retain analytes. In cases where the stationary phase is positively charged, anionic analytes will be retained. Likewise, with negatively charged stationary phases, cationic analytes will retain. (See 'Learn the Lingo' below). With IEX, proper selection of mobile phase pH is critical to ensure proper charge. Increasing the pH above the pKa will ionize weak acidic compounds while lowering the pH under the pKa will ionize weak basic ones.
- Hydrophilic Interaction (HILIC): This is the ‘reversed–reversed' mode, in which the stationary phase is “Normal Phase-like” and the mobile phase is “Reversed-Phase-like” – but in reversed proportions. Thus, using a polar stationary phase and a polar mobile phase containing a high proportion of water, HILIC's mechanism of action can be useful when separating highly polar compounds that are not well-retained in Reversed-Phase.
Learn the Lingo - Understanding IEX Shorthand
In cation-exchange chromatography, the stationary phase is negatively charged. In anion-exchange chromatography, the stationary phase is positively charged.
In IEX, the 1st letter refers to the stationary phase and means Strong or Weak. The 2nd letter describes the analyte – A for Anionic acidic compounds, and C for Cationic basic compounds. Thus:
- SCX - Strong Cation Exchange
- WCX - Weak Cation Exchange
- SAX - Strong Anion Exchange
- WAX - Weak Anion Exchange
Step Three: Choose the Chemistry
In chromatography, the key is to maximize the affinity between the analyte and the stationary phase, thus the most suitable stationary phase will be similar to the analytes. For this reason, you should choose a polar phase for polar analytes, a hydrophobic phase for hydrophobic analytes, an aromatic phase for aromatic analytes, and so on.

To improve your results, you could fine-tune a method using your usual stationary phase, but it would take a significant amount of time. Instead, changing the chemistry is often faster and more efficient to improve the selectivity.
- Reversed-Phase: The stationary phase is modified to be non-polar, typically by grafting hydrophobic chains (hydrocarbons) to interact with the analyte. Chromatographic columns are often named based on the length of the hydrophobic alkyl chains which are attached (e.g., C4 or C18).
Sorbent Structure Typical Applications C18 
Purification of low to high polarity compounds C8 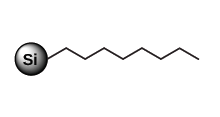
Less retention compared to C18, for highly hydrophobic & large molecule Cyclohexyl (C6) 
Alternative selectivity, with aromatic ring interactions, for purification of conjugated compounds (isomers) Pentafluorophenyl (PFP) 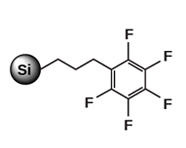
Moderately non-polar sorbent, for aromatic compounds Phenyl (PHE) 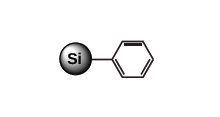
Moderately non-polar sorbent, for aromatic compounds C4 
Less retention compared to C18 and C8, for molecules with large hydrophobic regions C1 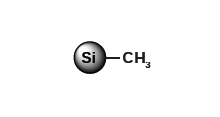
Less retention compared to other reversed-phases, for purification of polar and non-polar highly hydrophobic products Cyano (CN) 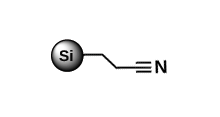
Versatile sorbent used either as normal or reversed-phase, less polar than silica - Normal Phase: the stationary phase can also be modified by grafting polar groups. Possible chemistries include:
Sorbent Structure Typical Applications Silica (Si) 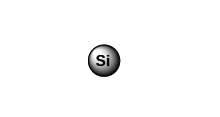
For purification of non-ionic polar organic compounds Diol nec 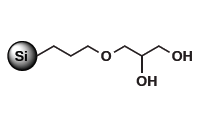
For difficult separation of low to medium polarity samples Amine (NH2 , WAX) 
For purification of compounds with basic properties - Ion Exchange (IEX): Depending on the phase, IEX can be used in a broad range of separations, including: sulfonic acids, acidic drugs, water-soluble vitamins, biomolecules, carboxylic acids, and basic drugs.
Sorbent Structure Typical Applications Amine (NH2 , WAX) 
- Weak anion exchanger (pKa of 9.8), positively charged at pH below 7.8
- For very strong anions (such as sulfonic acids), that may be too strongly retained on SAX phases
WAX-2 (Triethylamine) 
- Weak anion exchanger (pKa of 10.5), positively charged at pH below 8.5
- For compounds bearing a permanent negative charge (like salts of sulfonic acids)
SAX (TMA Chloride) 
- Strong anion exchanger, permanently positively charged (pH independent)
- For weak anions (such as carboxylic acids) that may not bind strongly enough on WAX phases
- Acetate counter-ion more easily exchangeable than the chloride one
SAX-2 (TMA Acetate) 
SCX (Tosic Acid) 
- Strong cation exchangers (pKa < 1), permanently negatively charged (pH independent)
- For purification of weak cations (basic drugs / analgesics, biomolecules & water-soluble vitamins)
SCX-2 (Propylsulfonic Acid) 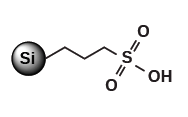
WCX (Carboxylic Acid) 
- Weak cation exchanger (pKa of 4.8), neutralized at pH below 2.8
- For strong cationic species, that may bind too strongly on SCX phases
- Hydrophilic Interaction (HILIC) : HILIC analytes are typically amino acids, nucleobases, alkaloids and nucleosides. Our Diol phase is commonly used in HILIC mode for biomolecule separations such as proteins and peptides, carbohydrates, glycosides and oligosaccharides.
For more in-depth information on phases and their applications

Check out pages 14 through 16 in this brochure:
« Solutions for Purification and Chromatography »
Another potential change in chemistry that should also be considered is whether to use an endcapped phase or not. Endcapping offers much lower residual silanols activity compared to non-endcapped silicas. What are some of those benefits?
- It avoids unwanted secondary interactions with free silanols on the surface. With functionalized silica, the only interaction that should occur is the one with the grafted function – and not a non-specific binding with the surface.
- When used in harsh conditions (with extreme pH, for example), endcapping prevents the silica surface from degradation.
Contact us today to find the right stationary chromatographic phase for your application
Or click here to learn more about SiliCycle's SiliaBond sorbents.





0 Comments